 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2017, Vol. 7, No. 43 doi: 10.5376/ijms.2017.07.0043
Received: 11 Oct., 2017 Accepted: 01 Nov., 2017 Published: 10 Nov., 2017
Hendri M., Rozirwan, and Apri R., 2017, Optimization of cultivated seaweed land Gracilaria sp using Vertikultur system, International Journal of Marine Science, 7(43): 411-422 (doi: 10.5376/ijms.2017.07.0043)
The production of Gracilaria sp is still not optimal and there are some opportunities left for production improvement by developing methods that use depth level as a planting media. A vertikultur method using bag nets is more effective and has higher production levels. This research aims to solve problems in cultivating Gracilaria sp seaweed by doing field optimization. Seaweeds were planted as many as 40 points for each 10 levels depth (BRL); 0, 70, 140, 210, 280, 350, 420, 560, and 630 cm from the surface. Environment and seaweed parameters were measured weekly. This research measures daily and weekly growth rate, absolute growth, and the total production. The data were analyzed using Anota test and BNJ further test. The research result shows that physic-chemistry water parameter is suitable for cultivating Gracilaria sp. The lowest weekly growth rate occurred in level BRL 10 in the first week while gaining weight as much as 9.351 gram/week. The highest growth rate occurred in level BRL 4 on the sixth week while gaining weight as much as 32.25 gram/week. The biggest absolute growth occurred in level BRL 4 with 214.87 gram and the lowest occurred in level BRL 10 with 161.45 gram. The biggest absolute growth rate occurred in level BR1 4 while gaining weight as much as 139.87 gram from the original weight and the lowest occurred in level BRL10 with 86.45 gram from the original weight. The best daily growth rate occurred in level BRL 4 with a growth rate of 2.53%/day and the lowest occurred in BRL 10 with 1.84%/day. Total production of Gracilaria sp from level BR1 1 – BR1 10 is 74,840 gram. The biggest production was obtained from level BRL 4 with the production of 8595 gram and the lowest in BRL 10 with 6458 gram. According to the anova test result and beda nyata jujur (BNJ), it shows that depth level (BR1 1 – BR1 10) gave no influence towards the growth rate of Gracilaria sp in the water of Kelagian Island. The vertikultur method by making use of depth level is both feasible and profitable for seaweed cultivators with production level ten times bigger.
Introduction
Seaweed cultivation is mostly done in water that is relatively calm and closed such as in a bay, behind an island. However, such waters have become too rare. Even if there is one, it usually has been used for cultivation of pearl, fish cages, seaweed, etc. Therefore, it is needed to make a breakthrough by doing an extension and intensification of the land for the cultivation to waters that have strong waves or by maximizing the land available by making use of water depth as media.
Gracilaria sp belongs to red algae group. This kind of algae is on of seaweed that is the most widely cultivated, its production is over 3.8 million ton/year with a value of US$ 1 billion/year (FAO, 2017). China and Indonesia are two biggest Gracilaria sp producer countries in the world (FAO, 2017). Gracilaria sp’s biomass is used for various purposes for the industrial sector such as industrial jelly (Pereira et al., 2008) and as animal feed (Qi et al., 2010; Johnson et al., 2014). Gracilaria sp contributes over 66% from the total production of jelly in the world (Pereira and Yarish, 2008). Until now, there are 185 kinds of Gracilaria sp that have been successfully identified (Guiry and Guiry, 2014). Gracilaria has a rapid growth rate (Abreu et al., 2011, Kim and Yarish, 2014; Kim et al., 2015; Wu et al., 2015; Kim et al., 2016; Gorman et al., 2017). This kind of seaweed has a high tolerance towards temperature (eurythermal). The range of temperature that could be tolerated ranged between 0 – 35°C with the optimum temperature for growth ranged between 20 – 28°C (Yokoya et al., 1999; Abreu et al., 2011; Kim et al., 2016). Moreover, Gracilaria sp has a wide tolerance towards salinity (euryhaline) ranged between 10 – 40 psu, with the optimum growth 25 – 33 psu (Yokoya et al., 1999; Weinberger et al., 2008; Kim et al., 2016; Gorman et al., 2017).
Gracilaria sp could be cultivated using various method such as longline method in the open sea, seabed, in a pond, and in a cultivation tank (Aslan, 1998; Oliveira et al., 2000; Indriani and Sumiarsih, 2005; Sahoo and Yarish, 2005; Pereira and Yarish, 2008). The success of cultivation depends on some factors which are; season, field’s compatibility, planting method, seeds and post-harvest. Some methods were used by seaweed cultivators in Indonesia such as seabed, longline, and ponds (Aslan, 1998; Indriani and Sumiarsih, 2005). The longline method needs a wide field. The use of wide fields costs a lot and the difficulty of maintaining the field could disturb cruise line. The existing method makes use of water surface as a planting media. This condition is very vulnerable to environment changes. Salinity and temperature fluctuation could lead to diseases. Waves and strong currents can cause thallus to break. This condition is different with water column that is relatively stable.
Meanwhile, the use of water column as planting media (vertikultur) has not been utilized. Vertikultur method is a seaweed planting method with a vertical way by using the depth (water column) as the media. Water depth that has been used could be varied depends on the penetration ability of the sunlight. This method is used while considering optimization, extension, and intensification of the field. The use of the method is expected to increase seaweed’s production. According to Aslan (1998), Darmawati (2013), Syahlun (2013), and Wisnu Ariyati et al. (2016), light penetration as the requirement of seaweed production could be obtained not only from one side of water surface but also from every angle of light elevation.
The depth of 30 – 120 cm gives a high response to growth (Safarudin, 2011). Seaweed could grow well because of the incoming sunlight into the water and the movements of the water that bring food substances that are needed by the seaweed. According to Susanto (2005), the growth of seaweed in the water with small waves is relatively smaller. This condition is caused by the attachment of substrate and epiphytic biota that could obstruct the growth (Wijayanto et al., 2014). The growth rate of seaweed in Lampung Bay using raft method is better than seabed and longline method (Pongarrang et al., 2013). On vertikultur method, the best planting space is 40cm with the original seed’s weight 100 gram. While according to (Herliany et al., 2016), the wider planting spacing will produce gross weight and relative growth that is bigger. Planting spacing 30 cm is better than 20 cm and 25 cm. Farman and Ilham (2015) stated that planting space has an influence on seaweed growth. The best planting spacing is 120 cm than 30 cm, 45 cm, 75 cm, 90 cm, and the original weight 100 gram in Sargassum sp cultivation using seabed method. While according to Darmawati (2013), the average daily growth rate in the depth of 50 cm (4.750%), higher than the daily growth rate in the depth of 20 cm (4.427%) and in the depth of 100 cm (3.892%).
Cultivation model that was developed in this research will use the combination of raft cultivation and vertikultur. Cultivated Seaweed is not tied but inserted in a kind of net pocket that is specially designed for preventing the break of thallus, fall out, and damaged by the waves, current, and predators. The purpose of this research is to analyze daily growth rate, specific growth rate, absolute growth rate, production and cultivation effectiveness of Gracilaria sp.
1 Materials and Methods
1.1 Research method
This research is conducted in Kelagian Island Waters in Lampung Province on September to November 2016.
1.2 Research design
The treatment given in this research is planting seaweed using a combination of depth level (vertikultur) as far as 10 levels with the code BRL1 – BRL10 as much as 40 repetition. Gracilaria sp seeds were inserted into the net pocket, planting space 70 cm (vertical) and 40 cm (horizontal). Vertikultur method using net pocket was designed resistant to the waves and strong current and could optimize the use of the field. The design of vertikultur method using net pocket Figure 1.
|
Figure 1 The Design of Vertikultur raft of Gracilaria sp seaweed |
Gracilaria sp seaweed seeds (BRL) that are planted is a young and good thallus. BRL were inserted into a 60 cm net pocket. Time spent for the maintenance is six weeks. The weighing of BRP is done every week.
1.3 Observed variable
Observed data in this research are:
(1) Water quality parameter including water physic parameter which covers: temperature, depth, current speed, brightness, and chemistry parameter which is salinity, nitrate, and phosphate.
(2) Weekly Growth Rate (WGR) is a weight on week ‘a’, (Wa) is the average weight ‘i’ (Ti) divided by the number of planting points (s) minus the previous week weight (Wb), with a formula:
.png)
.png)
(3) Absolute Growth Rate (AGR) is final weight (Wt) minus initial weight (Wo), with a formula:
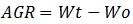
(4) Daily Growth Rate (DGR) is final weight (Wt) divided by initial weight (Wo) to the power of one/planting time (t) minus one multiplied by 100%, refers to Mtolera et al. (1995), Gerung and Ohno (1997), Aguirre-von-Wobeser et al. (2001), Bulboa et al. (2007), Hayashi et al. (2007), and Hori et al. (2009), with a formula:
.png)
(5) Total Production Rate is final weight level ‘i’ (Wl) minus initial weight level ‘i’ (Wo) plus the amount of cultivation depth level, with a formula:
.png)
The data collected were tested Normality (Steel and Torrie, 1993). Then, it will be processed using ANOVA test and BNJ further test.
2 Results and Discussion
2.1 Environment parameter
The result of measuring physic and chemistry factors of waters in the research location still decent for seaweed growth. Data is shown in Table 1.
|
Table 1 Physic and Chemistry Parameter of Waters |
Water temperature is around 29-30°C, with average temperature 29.64°C. there is no significant difference in the measurement of waters’ temperature during maintenance. The optimum temperature of seaweed growth is 20°C – 28°C (Aslan, 1998; Indriani and Sumiarsih, 2005). Gracilaria has a high tolerance towards temperature (0 – 35°C) (Yokoyama, 1999; Raikar et al., 2001; Abreu et al., 2011; Kim et al., 2016). The optimum temperature could improve nutrient absorption process so that it could hasten the growth rate. Seaweed has a varied range of temperature. High-temperature fluctuation could disturb metabolism and growth of Gracilaria sp. Excessive temperature fluctuation could be avoided using water mass movement (current).
Water salinity is around 31-32, with average salinity 31.57. the optimum salinity of seaweed growth is 32 ppm (Aslan, 1998; Indriani and Sumiarsih, 2005). Gracilaria has salinity tolerance 10 – 40 psu (Gorman and Zucker, 1997; Yokoyama, 1999; Klionsky et al., 2016). Seaweed has a specific range of varied salinity. Gracilaria sp is a kind of seaweed that has a wide range (euryhaline). Salinity really affects metabolism and growing process. pH value is around 7.40 – 8.00, with average 7.61. The optimum pH value of seaweed growth is around 7.5 – 8.0 (Aslan, 1998; Indriani and Sumarsih, 2005). The brightness of water is relatively same which is 100%. Sea water brightness is related to sunlight penetration into water area that is needed for photosynthesis process. The result of brightness measurement shows that 100% of sunlight has entered into the seabed. Water brightness decreased on the sixth week. This condition is caused by a small earthquake which makes the water a little murky.
The current in the research are is around 0.1 m/s – 0.2 m/s. The average current is around 0.15 m/s. The current has a big impact in the water mass change, aeration, nutrient transport and water mixing so that it affect the growth. Current also important and can be used to decrease the accumulation of sediment (silt) and epiphytic plants that stick on the thallus that can obstruct photosynthesis and growth process. A strong current can cause damage to thallus by breaking it and remove it. The current that is good for the cultivated seaweed is around the speed of 0.2 – 0.4 m/s (Indriani and Suminarsih, 2005). In calm water, the vegetation will get less nutrient so that it will disrupt the photosynthesis process.
On the research, location found that the seabed in the form of the sandy reef and the water is in form of windward. The concentration of nitrate and phosphate is around 0.35 mg/l and 0.0060 mg/l. Seaweed can grow to its optimum growth if the nitrate composition is around 0.9 – 3.5 mg/l (Atmadja et al., 1996), while the composition of phosphate is at optimum around 0.051 mg/l – 1.00 mg/l (Indriani and Suminarsih, 2005). The composition of nitrate and phosphate on the research location is at the level that it can sustain the growth of seaweed. Sulistijo (1996) said that the composition of phosphate that good for the seaweed is around 0.02 – 1 mg/l. Generally, those factors on the research location are in the perimeter for Gracilaria sp’s growth.
2.2 The growth of the seaweed
2.2.1 Weekly growth rate of Grasilaria sp seaweed
The growth rate of Grasilaria sp is measured once every seven days. Weekly weight gain of the seaweed is recorded in Table 2. The vertikultur method that has been in the application can be seen in Figure 2.
|
Table 2 Weekly Growth Rate of Gracilaria sp (WGR) |
|
Figure 2 Verticulture Methods |
The highest growth rate is dominated by shelf level BRL4 with the depth of maintenance point in 210 cm below the surface, while the lowest growth rate is in shelf level BRL10 with the depth of 630 below the surface (Table 2). The growth in the shelf level BRL4 is affected by several factors such as temperature, salinity, pH, nitrate and phosphate content is in the optimum condition for the metabolism and growth of Grasilaria sp.
The highest growth rate is in week 6 with 32.25 gram and the lowest is in week 1 with 9.35 gram (Table 3). The relatively slow growth which happens in week 1 is alleged because of the Grasilaria sp is still adapting to new environment. While the average growth rate in week 2 with 23.60 gram and the lowest with 13.88 (Week 1). The highest average growth rate occurs in shelf level BRL4 23.31 gram/week and the lowest is in BRL10 with 14.41 gram/week. The difference in growth rate of Grasilaria sp is affected by several factors such as temperature, salinity, sunlight, pH, and Do (Bold and Wynne, 1985; Pratiwi and Ismail, 2004). Seaweed needs the sunlight to do photosynthesis (Insan et al., 2013). The intensity of the sunlight will reduce as the depth level increase. Lombardi et al. (2006) stated that the brightness of sunlight can affect the growth of seaweed. A murky water contains sediment that can also affect the growth of seaweed. The sediment may stick to the thallus and obstruct the nutrient absorption and photosynthesis process. The depth is a factor that limits the growth rate (Kune, 2007). The intensity of the sunlight will reduce as the depth level increase.
|
Table 3 Weekly Relative Growth Rate |
The growth of Grasilaria sp is determined by the increase of weight and the number of thallus they have. The weight of the cultivation is an increase, increase in weight and the number of the thallus. The development of the research is good. The growth can be considered as good if the growth is increased by more than 2%/day. The growth in BRL1 – BRL9 is more than 2%/day, only in BRL10 is less than 2%/day. The growth can be considered as good if the daily growth is more than 2% (Ask and Azanza, 2002; Anggadiredja et al., 2006).
According to Erpin et al. (2013) and Widowati et al. (2015b), seaweed has a certain time span to reach their optimum growth. The growth tends to decrease after they reach their optimum growth. On week 6 the weather is not good, is cloudy, is rainy, and there is an earthquake that makes the water murky. Substrates that stick in the thallus will obstruct the growth and photosynthesis process. According to Susanto (2005) current and relatively weak wave can cause substrates to stick to the seaweed. The substrates that stick to the thallus can obstruct the sunlight and the nutrient absorption. This condition can affect the photosynthesis and metabolism process. With the photosynthesis and metabolism process is obstructed, it will also obstruct the optimum growth as well.
2.2.2 Absolute growth of the Grasilaria sp seaweed
The absolute growth of Grasilaria sp (Absolute Growth Rate) in this research is presented in Table 4. In the end of the research, the highest final weight occurs in the depth level of BRL4 with 214.87 gram. While the lowest is in BRL10 with 161.45 gram (Table 4). There is an increase of 2.15 – 2.86 times of the weight of the seaweed from the beginning of planting. The highest absolute growth rate occurs in level BRL4 with 139.87 gram and the lowest in level BRL10 with 86.45 gram.
|
Table 4 Absolute Growth Rate (AGR) of Grasilaria sp |
The highest growth rate in BRL4, allegedly in that depth level, the limiting factors such as nutrient (nitrate-phosphate), salinity, current, pH, temperature, and sunlight are at the optimum level to stimulate the growth rate of Grasilaria sp. The decrease in the growth rate because those factors have decreased and it will affect the growth as well.
Absolute growth rate tends to decrease as the depth increase. However, in BRL4 increased growth is occurred (Figure 3). The growth is also quite significant, it even is the highest growth rate of all BRL. This occurs because of several factors such as nutrient, temperature, pH, current, DO, and sunlight in the optimum condition. This research did not measure those factors base on the depth level. Measurement only occurs in BRL1 (surface). The sunlight as one of the instrument for Gracilaria sp’s growth can penetrate through the water until the seabed. However, with the number of BRL from BRL1 to BRL10, the intensity of the sunlight is decreased as it getsdeeper. Sunlight is one of the factors that dominates to affect the growth Gracilaria over the other factors.
|
Figure 3 Absolute growth rate of Gracilaria sp |
The depth level also affecting the growth of the seaweed. A result of the research done by Ponggarrang et al. (2013), in the depth of 40 cm has a better growth rate than in the depth of 20 cm and 30 cm. The intensity of the sunlight mostly affecting the growth of the Gracilaria sp. In the depth level that gets less sunlight, the growth will be slow. It is because the photosynthesis process is obstructed (Pratiwi and Ismail, 2004). Seaweed needs sunlight to do photosynthesis. Seaweed can grow in water with a certain depth with the sunlight that reaches the seabed. The result of the growth is affected by cultivation method, the absorption of sunlight for photosynthesis (Insan et al., 2013). The growth of seaweed will be apparent when there is an increasing number of the thallus and the increasing weight of the seaweed.
The optimal limit of the depth in verticultur method is 5 m (Pong-masak, 2010a). The best growth is in 2 m. in this research, the optimum condition for the growth to BRL10 is 630 cm and the best growth in the depth of 210 cm. The brightness of the water is up to 9 m. However, according to Pong-masak (2010a), it is in the 2 m depth. According to Insan et al. (2013), the best growth is in the depth of 30 cm and 60 cm rather than 90 cm. The growth of thallus will be more and fast.
2.2.3 Daily growth rate (DGR)
The daily growth rate of seaweed cultivation (BDRL) Gracilaria sp on every level is showing a good result (>2%/day) (Figure 4). The highest growth rate is in level BRL4 with the growth rate of 2.53%/day. While the lowest is in BRL10 with 1.84%/day (Table 5).
|
Figure 4 Daily growth rate of Gracilaria sp with vertikultur method |
|
Table 5 Daily Growth Rate (DGR) |
Daily growth in this research is categorized as good because the growth rate has qualified as good for seaweed cultivation. Growth rate can be considered good if the daily growth rate more than 2%/day (Ask and Azanza, 2002; Anggadiredja et al., 2006; Syahlun, 2013). There are several factors that can affect the growth rate of Grasilaria sp. (Lombardi et al., 2006), the brightness of the sunlight, the sediment that sticks to the thallus will obstruct the sunlight that is crucial for photosynthesis. Sunlight intensity, nutrient supply, depth also affect the growth of seaweed (Susilowati et al., 2012; Widowati et al., 2015a). The depth is one of the factors in determining the growth of the seaweed (Kune, 2007). With the increase of the depth the lower the sunlight the seaweed can get and the oxygen circulation is low. Also, the daily growth is affected by the maintenance time. Current and wave also affect the growth (Susanto, 2005). They can cause substrates to stick to the seaweed. The substrates that stick to the thallus can obstruct the sunlight and the nutrient absorption.
2.2.4 Production total rate (PTR)
Total production of the seaweed is the total weight Grasilaria sp seaweed and the harvest of the cultivation in 42 days (6 weeks). There is 40 planting point with 10 level of vertikultur depth each (BRL1 – BRL10) with the planting weight of 75 gram. However, the production rate is the difference between total productions and planting weight (Table 6).
|
Table 6 Production Total Rate (PTR) |
Total production that is produced by 10 level of depth with 40 points of planting with the weight of 74,840 gram (74.840 kg) and using 4.8 m2 (2 m x 2.4 m) land. The highest production is in the level BRL4 with 8,595 gram (8.595 kg) and the lowest is in BRL10 with 6,458 gram (6.458 kg). The production rate is 2.49 times more than the planting weight. Total production of conventional vertukultur (horizontal cultivation), whether using seabed, raft or long line method. The result is really good. If it using conventional will only produce 7,345 gram (7.345 kg) using 4.8 m2 (2 m x 2.4 m). While using vertikultur method with its benefit of depth will produce additional production from BRL2 – BRL10. The production rate can reach ten times more.
The production that earned is more than the research (Pong-masak, 2010b) that the harvest of vertikultur is five times more than any other method. Widowati et al. (2015a) gets the result three times more with the depth level of 30 cm, 60 cm, and 90 cm. Pongarrang et al. (2013) gets the result three times more with the depth level of 20 cm, 30 cm, and 40 cm. this method can be recommended for other cultivation with the result of bigger production. This method also can be used in the wavy and strong current water. The concern of the broken thallus of Gracilaria sp can be avoided by planting it on the water column and using the net pocket.
2.2.5 Data analysis of seaweed growth effectiveness
To test the effectiveness of the relative growth rate can be done by comparing the growth rate of the seaweed of each sample, do the normality test first.
Based on the result of the normality test, Shapiro will show that the data is distributed normally with sig >0.05. After that, the data is processed by using anova test. The result of anova test shown that F count is smaller than the F Table (F count 0.191 < F Table 2.08). The data above show that the H1 is rejected with the consequence of H0 accepted or there is no effect of the depth level (BRL) to the growth of the seaweed.
Next is the advance beda nyata jujur (BNJ) test. Based on the test, there is no significant data on the depth, BRL1 – BRL10. The result will be drawn by looking at the significance value and symbol (*) in mean difference. The symbol that was used is a, b, c, d and so on. The data did not show any significant whatsoever. It means that the data did not affect the depth level (BRL1 – BRL10) and the growth of the Gracilaria sp in Kelagian Island’s water. The result did not show that the depth level affects the growth of Gracilaria sp which supported by the average growth in each depth level >2%/day. According to Ask and Azanza (2002), Anggadiredja et al. (2006), and Syahlun (2013), the growth that shows >2%/day is the best growth rate and is recommended for seaweed cultivation. Only in BRL10, the growth rate is <2%/day. Based on the data, the growth rate and the data analysis, the Gracilaria sp seaweed cultivation in Kelagian Island’s water is probable to plant the seaweed up to the level of BRL10 (630 cm below the surface).
3 Conclusions
The conclusions of the research are:
The physics-chemistry parameter of the water of seaweed cultivation E.cottonii vertikultur method in Kelagian island still in the optimal limit for the growth of seaweed cultivation of E.cottonii.
The lowest weekly growth rate is in BRL10 by the first week with additional weight of 9,351 gram/week. The highest growth rate is in level BRL4 in the sixth week additional weight of 32.25 gram/week.
The highest absolute growth is in level BRL4 with the weight of 214.87 gram and the lowest is in level BRL10 with the weight of 161.45 gram. The highest absolute growth rate is in BRL4 with the additional weight of 139.87 gram from the planting weight and the lowest is in BRL10 with the additional weight of 86.45 gram from the planting weight.
The best daily growth rate (DGR) is in level BRL4 with the growth rate of 2.53%/day and the lowest is in BRL10 with 1.84%/day.
Production total of Gracilaria sp from BRL1 – BRL10 by 74,840 gram. The biggest production is gain from BRL4 by 8,595 gram of production and the lowest is in BRL10 by 6,458 gram.
Based on anova test and beda ntyata jujur (BNJ) show that there is no effect done to the depth level (BRL1 – BRL10) to the growth rate of Gracilaria sp in the Kelagian Island’s waters.
The vertikultur method by using the depth level can be done and profitable for seweed cultivation.
This research sugests that:
For opimization of the land, Gracilaria sp cultivation can use vertikultur method with the depth level up to 630 cm (BRL1 – BRL10).
The measurement of the water’s parameter preferably done in each depth level to see its effect to the growth.
Authors’ contributions
Thank Rozirwan for his contributions during his research especially abstract, editing and other writing. Also to Rezi Apri for contributions during the research, opinions for the improvement of this research and writing.
Acknowledgments
Thanks to, Sahala Tua Batubara and Yonathan Sinaga for their contributions during the research. This research was financed from DIPA of Sriwijaya University Budget No. 042.01.2.400953/2016 Date December 7, 2016 In accordance with the Letter of Appointment Agreement Implementation Research Competitive University of Sriwijaya Number 592 / UN9.3.1 / LT / 2016. April 22, 2016.
Abreu M.H., Pereira R., Buschmann A., Sousa-Pinto I., and Yarish C., 2011, Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium, Journal of Experimental Marine Biology and Ecology, 407(2): 190-199
https://doi.org/10.1016/j.jembe.2011.06.034
Aguirre-von-Wobeser E., Figueroa F., and Cabello-Pasini A., 2001, Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines, Marine Biology, 138(4): 679-686
https://doi.org/10.1007/s002270000506
Anggadiredja J., Zatnika A., Purwoto H., and Istini S., 2006, Rumput Laut, Penebar Swadaya, Jakarta, 152: 54-59
Ask E.I., Azanza R.V., 2002, Advances in cultivation technology of commercial Eucheumatoid species: a review with suggestions for future research, Aquaculture, 206(3): 257-277
https://doi.org/10.1016/S0044-8486(01)00724-4
Aslan L.M., 1998, Rumput Laut, Yogyakarta: Kanisius
Atmadja W., Kadi A., and Sulistijo R., 1996, Pengenalan jenis-jenis rumput laut Indonesia, Puslitbang Oseanologi, LIPI, Jakarta85, Introduction to the algae, Prentice-Hall, inc., Englewood Cliffs, New Jersey, USA, pp.720
Bulboa C.R., de Paula E.J., and Chow F., 2007, Laboratory germination and sea out-planting of tetraspore progeny from Kappaphycus striatum (Rhodophyta) in subtropical waters of Brazil, Journal of Applied Phycology, 19(4): 357-363
https://doi.org/10.1007/s10811-006-9142-7
Darmawati D., 2013, Analisis laju pertumbuhan rumput laut Kappaphycus alvarezii yang ditanam pada berbagai kedalaman, Octopus: Jurnal Ilmu Perikanan, 2(2): 184-191
FAO, 2017, The state of world fisheries and aquaculture, Accessed January 23
Farman A., and Ilham I., 2015, Budidaya rumput laut Sargassum sp menggunakan metode lepas dasar dengan jarak tanam yang berbeda, Buletin Teknik Litkayasa Akuakultur, 13(2): 137-142
http://dx.doi.org/10.15578/blta.13.2.2015.137-142
Gerung G.S., and Ohno M., 1997, Growth rates of Eucheuma denticulatum (Burman) Collins et Harvey and Kappaphycus striatum (Schmitz) Doty under different conditions in warm waters of Southern Japan, Journal of Applied Phycology, 9(5): 413-415
https://doi.org/10.1023/A:1007906326617
Gorman L., Kraemer G.P., Yarish C., Boo S.M., and Kim J.K., 2017, The effects of temperature on the growth rate and nitrogen content of invasive Gracilaria vermiculophylla and native Gracilaria tikvahiae from Long Island Sound, USA, Algae, 32(1): 57-66
https://doi.org/10.4490/algae.2017.32.1.30
Gorman M., and Zucker I., 1997, Environmental induction of photo non responsiveness in the Siberian hamster, Phodopus sungorus, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 272(3): 887-895
Guiry M., and Guiry G., 2014, AlgaeBase, World-wide electronic publication, National University of Ireland, Galway
Hayashi L., de Paula E.J., and Chow F., 2007, Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State, Brazil, Journal of Applied Phycology, 19(5): 393-399
https://doi.org/10.1007/s10811-006-9135-6
Herliany N.E., Zamdial Z., and Meylia R., 2016, Cultivation of seaweed Gracillaria sp. using longline methods under different space of planting, Journal of Aquatropica Asia, Vol 2, No 2
h rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam, Journal of Applied Phycology, 21(3): 265-272
https://doi.org/10.1007/s10811-008-9360-2
Indriani H., and Sumiarsih E., 2005, Rumput Laut. Jakarta: Penebar Swadaya
Insan A.I., Widyartini D.S., and Sarwanto S., 2013, Posisi tanam rumput laut dengan modifikasi sistem jaring terhadap pertumbuhan dan produksi Eucheuma cottonii di perairan Pantura Brebes, Jurnal Litbang Provinsi Jawa Tengah, 11(1): 125-133
Johnson R.B., Kim J.K., Armbruster L.C., and Yarish C., 2014, Nitrogen allocation of Gracilaria tikvahiae grown in urbanized estuaries of Long Island Sound and New York City, USA: a preliminary evaluation of ocean farmed Gracilaria for alternative fish feeds, Algae, 29(3): 227
Kim J.K., Mao Y., Kraemer G., and Yarish C., 2015, Growth and pigment content of Gracilaria tikvahiae McLachlan under fluorescent and LED lighting, Aquaculture, 436: 52-57
https://doi.org/10.1016/j.aquaculture.2014.10.037
Kim J.K., and Yarish C., 2014, Development of a sustainable land-based Gracilaria cultivation system, Algae, 29(3): 217
https://doi.org/10.4490/algae.2014.29.3.217
Kim J.K., Yarish C., and Pereira R., 2016, Tolerances to hypo-osmotic and temperature stresses in native and invasive species of Gracilaria (Rhodophyta), Phycologia, 55(3): 257-264
https://doi.org/10.2216/15-90.1
Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., and Adeli K., 2016, Guidelines for the use and interpretation of assays for monitoring autophagy, Autophagy, 12(1): 1-222
http://dx.doi.org/10.1080/15548627.2015.1100356
Kune S., 2007, Pertumbuhan rumput laut yang dibudidaya bersama ikan Baronang, Jurnal Agrisistem, 3(1): 7-9
Lombardi J.V., de Almeida Marques H.L., Pereira R.T.L., Barreto O.J.S., and de Paula E.J., 2006, Cage polyculture of the Pacific white shrimp Litopenaeus vannamei and the Philippines seaweed kappaphycus alvarezii, Aquaculture, 258(1): 412-415
Mtolera M.S., Collén J., Pedersén M., and Semesi A.K., 1995, Destructive hydrogen peroxide production in Eucheuma denticulatum (Rhodophyta) during stress caused by elevated pH, high light intensities and competition with other species, European Journal of Phycology, 30(4): 289-297
http://dx.doi.org/10.1080/09670269500651071
Oliveira E.C., Alveal K., and Anderson R.J., 2000, Mariculture of the agar-producing Gracilarioid red algae, Reviews in Fisheries Science, 8(4): 345-377
http://dx.doi.org/10.1080/10408340308951116
Pereira R., Kraemer G., Yarish C., and Sousa-Pinto I., 2008, Nitrogen uptake by gametophytes of Porphyra dioica (Bangiales, Rhodophyta) under controlled-culture conditions, European Journal of Phycology, 43(1): 107-118
http://dx.doi.org/10.1080/09670260701763393
Pereira R., and Yarish C., 2008, Mass production of marine macroalgae, Encyclopedia of Ecology, Ecological Engineering, Elsevier, 3: 2236-2247
Pong-masak R., 2010a, Panen 10 Kali Lipat dengan Vertikultur, Majalah TROBOS Juni 2010
Pong-masak R., 2010b, Panen 10 Kali Lipat dengan Vertikultur, Majalah TROBOS Edisi Juni 2010, Diakses 18-09-2010, Hlm 1
Pongarrang D., Rahman A., and Iba W., 2013, Pengaruh jarak tanam dan bobot bibit terhadap pertumbuhan rumput laut (Kappaphycus alvarezii) menggunakan metode vertikultur, Jurnal Mina Laut Indonesia, 3(12): 94-112
Pratiwi E., and Ismail W., 2004, Perkembangan budidaya rumput laut di Pulau Pari, Warta, 2: 11-15
Qi Z., Liu H., Li B., Mao Y., Jiang Z., Zhang J., and Fang J., 2010, Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus Hannai Ino, Aquaculture, 300(1): 189-193
https://doi.org/10.1016/j.aquaculture.2010.01.019
Raikar S., Iima M., and Fujita Y., 2001, Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India
http://hdl.handle.net/123456789/4608
Sahoo D., and Yarish C., 2005, Mariculture of seaweeds, Phycological Methods: Algal Culturing Techniques, Academic Press, New York, 219-237
Steel R.G., and Torrie J.H., 1993, Prinsip dan Prosedur Statistika
Sulistijo D.W.A., 1996, Pertumbuhan alga laut Eucheuma spinosum pada berbagai kedalaman, Oseanologi Indonesia
Susanto A., 2005, Metode lepas dasar dengan model cidaun pada budidaya Eucheuma spinosum (Linnaeus) Agardh, Indonesian Journal of Marine Sciences, 10(3): 158-164
https://doi.org/10.14710/ik.ijms.10.3.158-164
Susilowati T., Rejeki S., Zulfitriani Z., and Dewi E.N., 2012, The influence of depth of plantation to the growth rate of Eucheuma cottonii seaweed cultivated by longline method in Mlonggo beach, Jepara Regency, Jurnal Saintek Perikanan, 8(1): 7-12
https://doi.org/10.14710/ijfst.8.1.7-12
Syahlun R.A.R., 2013, Pertumbuhan rumput laut (Kappaphycus alvarezii) strain coklat dengan metode vertikultur, Jurnal Mina Laut Indonesia, 1(0): 1
Weinberger F., Buchholz B., Karez R., and Wahl M., 2008, The invasive red alga Gracilaria vermiculophylla in the Baltic Sea: adaptation to brackish water may compensate for light limitation, Aquatic Biology, 3(3): 251-264
https://doi.org/10.3354/ab00083
Widowati L.L., Rejeki S., Yuniarti T., and Ariyati R.W., 2015a, Efisiensi produksi rumput laut E. cotonii dengan metode budidaya long line vertikal sebagai alternatif pemanfaatan kolom air, Jurnal Saintek Perikanan, 11(1): 47-56
https://doi.org/10.14710/ijfst.11.1.47-56
Widowati L.L., Rejeki S., Yuniarti T., Ariyati R.W., 2015b, Efisiensi Produksi rumput laut E.cottonii dengan metode long line vertikal sebagai alternatif pemanfaatan kolom air, Jurnal Saintek Perikanan, 11(1): 47-56
https://doi.org/10.14710/ijfst.11.1.47-56
Wijayanto T., Hendri M., and Aryawati R., 2014, Studi pertumbuhan rumput laut Eucheuma cottonii dengan berbagai metode penanaman yang berbeda di perairan Kalianda, Lampung Selatan, Maspari Journal, 3(2): 51-57
Wisnu Ariyati R., Lakhsmi Widowati L., and Rejeki S., 2016, Performa produksi rumput laut Euchema cottonii yang dibudidayakan menggunakan metode long-line vertikal dan horisontal
Wu H., Huo Y., Han F., Liu Y., and He P., 2015, Bioremediation using Gracilaria chouae co-cultured with Sparus macrocephalus to manage the nitrogen and phosphorous balance in an IMTA system in Xiangshan Bay, China, Marine pollution bulletin, 91(1): 272-279
https://doi.org/10.1016/j.marpolbul.2014.11.032
Yokoya N.S., Kakita H., Obika H., and Kitamura T., 1999, Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan, Hydrobiologia, 398: 339-347
https://doi.org/10.1023/A:1017072508583
Yokoyama S., 1999, Molecular bases of color vision in vertebrates, Genes & Genetic Systems, 74(5): 189-199
. PDF(843KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Muhammad Hendri
. Rozirwan
. Rezi Apri
Related articles
. Vertikultur
. Kelagian Island
. Seaweed
. Gracilaria sp
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)


